Dr. Edgley is the Director of Stroke Rehabilitation at the University of Utah and currently participating as a site principle investigator for the Ipsen ABOLISH study. This is an international observational study to assess the longitudinal attainment of personal centered and function related goals after one or more abobotulinum toxin A (aboBoNT-A) injections to treat lower limb spasticity using the cumulated Goal Attainment Scaling-Leg (GASleg) T score in a real life clinical setting. Spasticity is one of the most common and disabling conditions associated with many neurological diseases in adults. Spasticity is characterized by the co-occurrence of paresis and involuntary velocity-dependent muscle hyperactivity due to exaggerated stretch reflexes, resulting in greater resistance to passive movement. Spasticity arises as a result of damage to the central nervous system and is one of many signs of upper motor neuron (UMN) syndrome. Potential Participants will be considered if they are between the ages of > 18 years of age, primary diagnosis of unilateral adult lower limb spasticity, subject can take more than five steps with or without assistance with the decision to treat lower limb with aboBoNT-A by their provider/clinician.
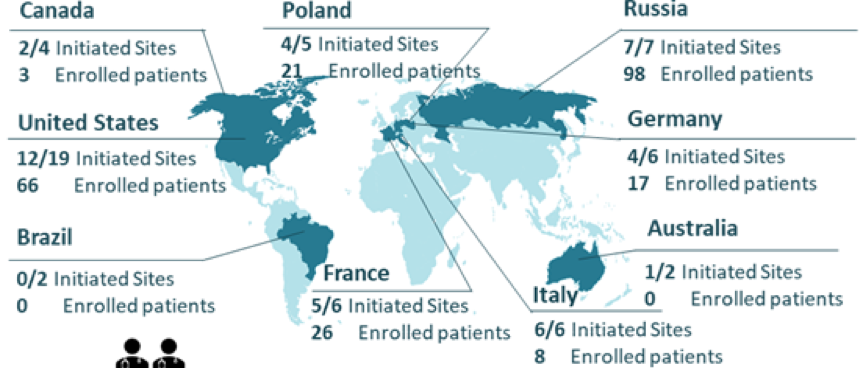
Clinical Research as a whole has been impacted by the COVID-19 pandemic worldwide. The deadline for the last patient enrollment is March 2021, we are working hard to help reach the sponsor’s target March deadline. The University of Utah would like to enroll at least eight more participants by March.